Introduction to Science:
Have you ever wondered: "How did The Universe form?", "Why diamonds are so hard?", "Why do we cough?" or anything like that? Well, you can get the answers to all those questions through science!
Definition:
"The knowledge that provides an understanding of this Universe and how it works is called "Science". OR
"The knowledge gained through observations and experiments is called "Science".
Simplified Definition:
The knowledge that tells us how this Universe works is called "Science".Etymology:
The word "Science" is derived from the Latin word "Scientia" which means "knowledge" and it is taken from the world "Scire" (pronounced as "Shee-eray") which means "to know".
Brief History:
At first, the properties of materials were studied under a single branch called "Natural Philosophy". But, as the knowledge increased it was divided into branches. In the eighteenth century, natural philosophy was divided into "Physical sciences" and "Biological sciences" or "Life sciences".
Branches:
The five main branches of science are:
1.) "Physical sciences" (the study of non-living things)
2.) "Biological sciences" or "Life sciences" (the study of living things)
3.) "Social sciences" (the study of society)
4.) "Formal sciences" (the study of logic and numbers etc)
5.) "Applied sciences" (the study of using and applying science)
These branches are divided into more and more branches.
This blog is created to help you in understanding "Chemistry", which is a branch of Physical sciences.
Introduction to Chemistry:
When we look at different things, many questions arise in our mind e.g. "What is this?", "What is it made up of?" and "How was this thing made?" etc. Well, Chemistry helps us in understanding what something is made up of.
Definition:
The branch of science that deals with the study of composition, properties, changes, and structure of matter (anything that takes up space) is called "Chemistry" or "Chemical Science".
Simplified Definition:
The branch of science in which we learn: "What is matter made up of?", "What things make up matter?", "How those things are arranged?", "What are their properties?", "How do they change?" etc is called "Chemistry" or "Chemical Science".
Atoms:
The main focus of chemistry is on atoms and how they react to form and bring changes in matter. Atoms are sometimes called the "basic building blocks" of matter.
Definition:
"An atom is the smallest particle of a substance that can exist by itself or be combined with other atoms to form a molecule."
Simplified Definition:
"An atom is the smallest piece of matter that takes up space, joins with other atoms to form molecules which in turn combine us and everything that is around us."
Explanation:
In chemistry, we learn that anything that takes up space is made up of them or forms them, whether the thing is small or big, soft or hard, solid or gas, round or irregular in shape or whatever! In a building (whether small or tall, strong or weak, old or new), bricks are the smallest units and similarly, in matter, atoms are the smallest units. Or just like how all puzzles are made up of small individual pieces of puzzles and all kinds of LEGO shapes are made up of small LEGO pieces, atoms are the smallest individual units that make up all matter. But, don't forget that they are WAY smaller than puzzle pieces and these are just examples to get an idea. Atoms don't have a definite shape but usually seem spherical under microscopes and are depicted as spheres in books etc. They cannot be broken down into simpler parts by chemical means. Solids, liquids, gases, and plasma are all made up of atoms. Almost EVERYTHING is made up of atoms.Size:
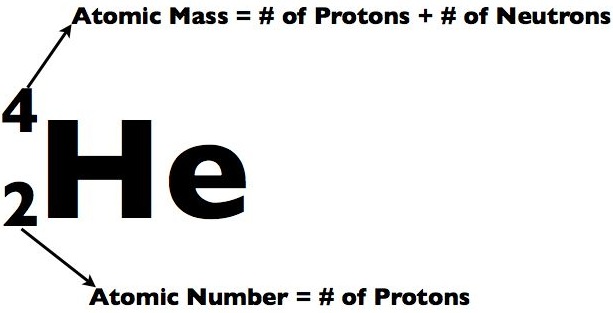
Typically, an atom's size is 100pm (pico meter - a very small mass measuring unit). That's about tenth-billionth of a meter. They are very very small and can only be seen with very powerful microscopes such as Electron Tunneling Microscopes (ETM) and Atomic Force Microscopes (AFM).
There are about a million atoms in the width of a human hair.
Sub-Particles:
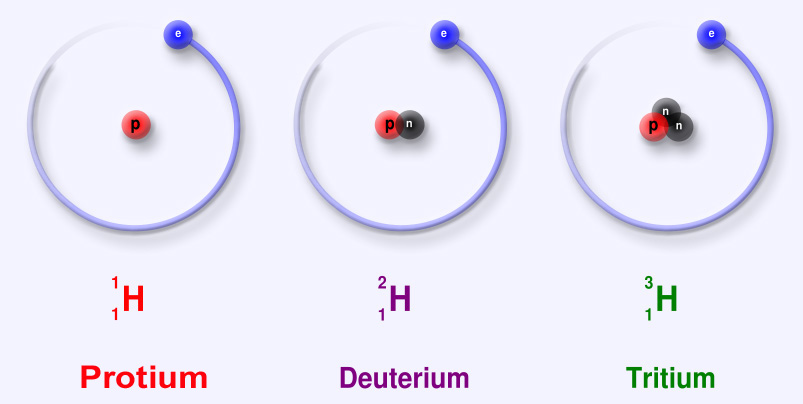
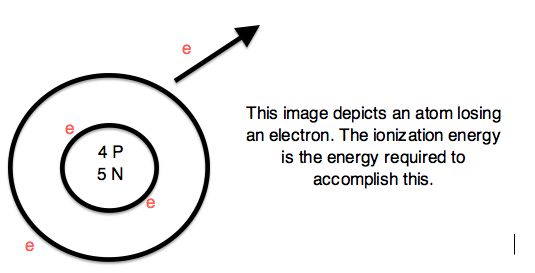
Sub-particles are further and smaller particles of an atom. They form the structure of an atom. They form the "Nucleus". Any and all particles inside the nucleus are called "Nucleons". It contains particles called protons and neutrons together. Protons have a positive (+) charge and Neutrons have 0 or no charge (same thing).
Mass of proton = 1.0072766 a.m.u. or 1.6726 x 10 to the power -29 kg
and
Mass of neutron = 1.0086654 a.m.u. or 1.6749 x 10 to the power of -27 kg
Over 99.94% of the atom's mass is in the nucleus. Around the nucleus, negatively charged (-) very very small particles called "electrons" whiz (not orbit in "perfectly circular" paths, It is said to make it easier for the beginners) around the nucleus in "Shells" (sort of paths also called orbits or energy levels) which are divided into "Sub-shells". Sub-shells are further divided into "Orbitals". In an orbital, every two electrons spin (rotate like a top on their "axis") in opposite directions. Electrons have 3 parts. Spinon gives an electron it's spin, Holon gives it it's charge and Orbiton helps it to orbit around the nucleus. These are the quasiparticles of the electrons.
Mass of electron = 0.000548597 a.m.u. or 9.1 x 10-31 kg
A proton is about 1837 times larger than an electron's and a neutron is about 1842 times larger than an electron.
Protons: "p"
Neutron: "n".
Mass of proton = 1.0072766 a.m.u. or 1.6726 x 10 to the power -29 kg
and
Mass of neutron = 1.0086654 a.m.u. or 1.6749 x 10 to the power of -27 kg
Over 99.94% of the atom's mass is in the nucleus. Around the nucleus, negatively charged (-) very very small particles called "electrons" whiz (not orbit in "perfectly circular" paths, It is said to make it easier for the beginners) around the nucleus in "Shells" (sort of paths also called orbits or energy levels) which are divided into "Sub-shells". Sub-shells are further divided into "Orbitals". In an orbital, every two electrons spin (rotate like a top on their "axis") in opposite directions. Electrons have 3 parts. Spinon gives an electron it's spin, Holon gives it it's charge and Orbiton helps it to orbit around the nucleus. These are the quasiparticles of the electrons.
Mass of electron = 0.000548597 a.m.u. or 9.1 x 10-31 kg
A proton is about 1837 times larger than an electron's and a neutron is about 1842 times larger than an electron.
Symbols:
Electron: "e"Protons: "p"
Neutron: "n".
Note: The following information in this post is related to Physics and not Chemistry. You may skip it if you want.
Quarks:
Inside the protons and neutrons, there are smaller particles called "Quarks". Their symbol is "q". Quarks are held together by neutral particles called "Gluons". They are are named that because they kind of "glue" the quarks very strongly (in easy words). "On" is the usual particle suffix i.e. a word added after their name.
There are 6 types of quarks. These "types" are called the "flavors" of quarks. There is a group of particles called "Hadrons" which are particles made up of quarks. Protons consist of 2 up quarks and 1 down quark held together by the gluon and Neutrons consist of 2 down quarks and 1 up quark held together by the gluon. This is what gives them their properties and identity. No other particles have these combinations of quarks.
1.) Up quark: 1 attometer OR 1x10 to the power of -18
2.) Down quark: 1 attometer OR 1x10 to the power of -18
3.) Strange quark: 400 zeptometers or 4x10 to the power of -19 meters
4.) Charm quark: 100 zeptometers or 1x10 to the power of -19
5.) Bottom quark: 30 zeptometers or 3x10 to the power of -20 meters
6.) Top quark: 100 yoctometers or 1x10 to the power of -22
We can easily use simple math to do this:
Charge of a proton = Charge of 2 Up quarks + Charge of 1 down quark + Charge of Gluon
= (2/3 + 2/3) + (-1/3) + 0
= 2/3 + 2/3 - 1/3 + 0
= (2+2-1+0)/3
= 3/3
= 1 Answer
Hence, the charge on a proton is +1!
Now, here's why neutrons have 0 charge:
Neutrons are made up of 1 up quark and 2 down quarks held together by the gluon.
Charge of a neutron = Charge of 1 Up quark + Charge of 2 Down quarks + Charge of the gluon
= (+2/3) + (-1/3) + (1/3) + 0
= 2/3 -1/3 -1/3 + 0
= (2-1-1+0)/3
= 0/3 (Any number divided by 0 is equal to 0.)
= 0 Answer
And that's why they have 0 or no charge!
There are 6 types of quarks. These "types" are called the "flavors" of quarks. There is a group of particles called "Hadrons" which are particles made up of quarks. Protons consist of 2 up quarks and 1 down quark held together by the gluon and Neutrons consist of 2 down quarks and 1 up quark held together by the gluon. This is what gives them their properties and identity. No other particles have these combinations of quarks.
Masses:
.The masses of quarks are not confirmed. Their names and masses are:1.) Up quark: 1 attometer OR 1x10 to the power of -18
2.) Down quark: 1 attometer OR 1x10 to the power of -18
3.) Strange quark: 400 zeptometers or 4x10 to the power of -19 meters
4.) Charm quark: 100 zeptometers or 1x10 to the power of -19
5.) Bottom quark: 30 zeptometers or 3x10 to the power of -20 meters
6.) Top quark: 100 yoctometers or 1x10 to the power of -22
Charges:
1.) Up, Top and Charm quarks, all have a charge of +2/3
2.) Down, Bottom and Strange, all have a charge of -1/3
2.) Down, Bottom and Strange, all have a charge of -1/3
Why do protons have a positive charge and neutrons have no charge?
Protons are made up of two up quarks and one down quark held together by the gluon (neutral). These particles make up a proton. That means if we add the sum of their charges, we get the charge of the proton!We can easily use simple math to do this:
Charge of a proton = Charge of 2 Up quarks + Charge of 1 down quark + Charge of Gluon
= (2/3 + 2/3) + (-1/3) + 0
= 2/3 + 2/3 - 1/3 + 0
= (2+2-1+0)/3
= 3/3
= 1 Answer
Hence, the charge on a proton is +1!
Now, here's why neutrons have 0 charge:
Neutrons are made up of 1 up quark and 2 down quarks held together by the gluon.
Charge of a neutron = Charge of 1 Up quark + Charge of 2 Down quarks + Charge of the gluon
= (+2/3) + (-1/3) + (1/3) + 0
= 2/3 -1/3 -1/3 + 0
= (2-1-1+0)/3
= 0/3 (Any number divided by 0 is equal to 0.)
= 0 Answer
And that's why they have 0 or no charge!