Unified Atomic Mass:
The unified atomic mass unit (symbol: u) or dalton (symbol: Da) is the standard unit that is used for indicating mass on an atomic or molecular scale (atomic mass). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
Relative Atomic Mass:
The relative atomic mass (symbol:
Ar) of an atom is the average mass of an atom as compared to 1/12th the mass of a Carbon-12 isotope.It is also called "Atomic Weight". Based on carbon-12 standard, the mass of a carbon atom is 12 and 1/12th of it comes out to be one. It's unit is Atomic Mass Unit (amu). When atomic mass unit is expressed in grams, it is 1 amu = 1.66 x 10 to the power of -24 grams.
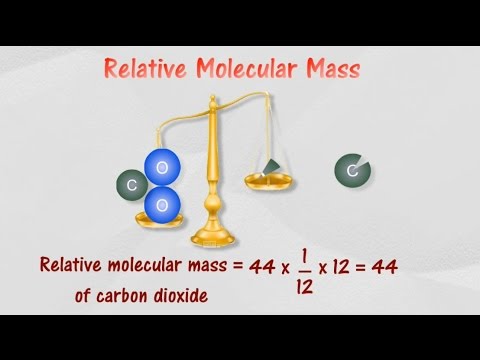
Relative Molecular Mass:
The average mass of a molecule as compared to 1/12th of a Carbon-12 isotope is called "Relative Molecular Mass". It is also called "molecular weight", "relative molar mass", "molar weight", "formula mass" and "formula weight". The symbol for relative molecular mass is Mr . It is a dimensionless quantity. It has no units. It is the sum of all relative atomic masses of atoms in a molecule.
Relative Formula Mass:
The average mass of a formula unit (e.g. one NaCl in salt) as compared to 1/12th of a Carbon-12 isotope is called "Relative Formula Mass". The symbol for relative formula mass is aslo Mr . It is the sum of all relative atomic masses of atoms in a formla unit.
Relative Isotopic Mass:
Relative isotopic mass is the mass of an atom of an isotope compared with one-twelfth the mass of a carbon-12 atom.
 |
| Equation of Unified Atomic Mass |
 |
| Amu |
 |
| Relative Molecular Mass |


No comments:
Post a Comment