Atomic Size/Atomic Radius:
The distance from the nucleus to the outermost shell is called "Atomic Radius" OR The radius of an atom is called "Atomic Radius". It decreases from left to right in a period and increases from up to down in a group. For example 265 pm (pico meter) is the atomic radius of Cesium.
Electronegativity:
In easy words, it is the "how much an atom attracts a bonding pair of electrons".. It increases from left to right in a period and decreases from up to down in a group. For example: The electronegativity of Flourine is 4.0, the highest.
Ionization Energy:
The amount of energy needed to remove an electron from the outermost shell is called "Ionization Energy". The energy needed to remove the 2nd electron from a shell is called "2nd Ionization Energy" and so on. It increases from left to right in a period and decreases from up to down in a group. For example: Ionization energy of H is 1312.0.
Electron Affinity:
Energy is released when an electron enters a shell. The amount of energy released when an electron is added in the outermost shell is called "Electron Affinity". It increases from left to right in a period and increases from down to up in a group. For example: Silicon's electron affinity is 74 kilo joules per mole.
Shielding Effect:
The protons of the nucleus exerts force of attraction. The electrons of the shell that is the closest to the nucleus (K-shell) are attracted with most force. These electrons shield the force of attraction a bit. So, the electrons of the upper shell (L-shell) are attracted with a bit lesser force than the electrons of K-shell. These electrons also shield some force. This is done by electrons in all shells. So, the electrons of the outermost shell are attracted with very low force. This effect is called "Shielding Effect" and the electrons that shield the force of attraction are called "Shielding Electrons". It increases from up to down in a group and has no effect as you move from left to right in a period.
 |
| Atomic Radius |
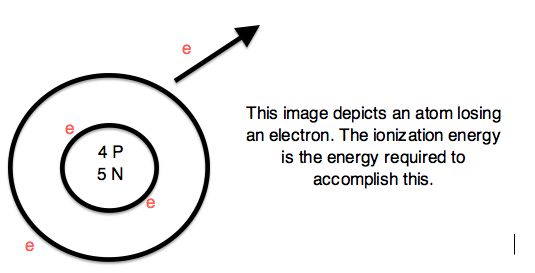 |
| Ionization Energy |
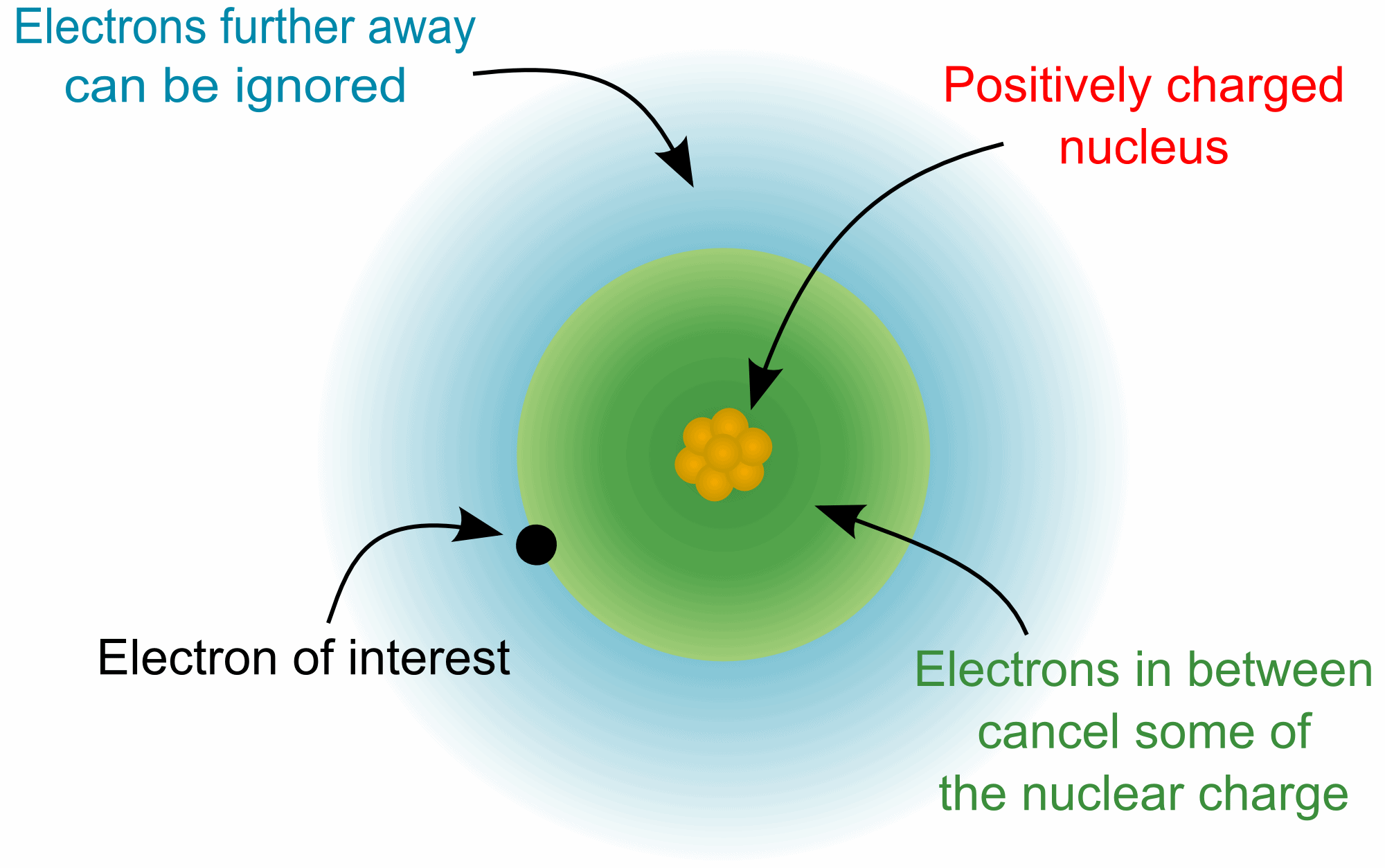 |
| Shielding Effect |
3 comments:
Mesmerized article written on this blog with other relevant information. It is straight to the point that how we can improve our skills as well as how we can be represented to a new stream of professionalism.FORTIANALYZER 400E
The official application of Uptodown for Android operating systems. Quickly access descriptions, screenshots, and of course, complete apps for Android
CASINO - 15406 S. Main St, Phoenix, AZ 85339
Find out what's popular at Casino 부천 출장안마 at 울산광역 출장안마 15406 통영 출장마사지 S Main St, Phoenix, AZ 순천 출장안마 85339. See activity. 동해 출장마사지
Post a Comment