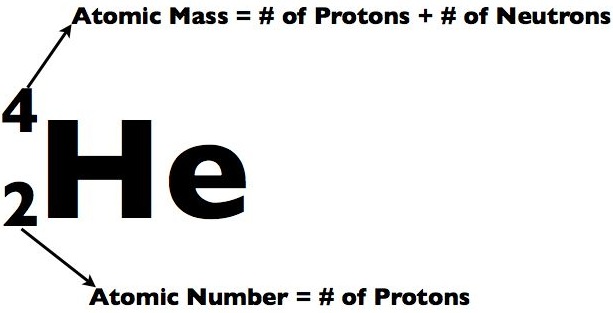
Atomic Number:
The number of protons in an atom is called Atomic Number of that atom e.g. Hydrogen contains 1 proton. So, it's atomic number is 1. Carbon contains 6 protons. So, it's atomic number is 6. The symbol of Atomic Number is Z. For example: For Helium, Z = 2 because Helium has 2 protons in it's nucleus. All atoms of an element have the same number of protons because they are same types of atoms. So, they have the same atomic number. Different elements have different atomic numbers.
Atomic Mass and Mass Number:
Atomic Mass (Symbol: m) is the average mass of an atom of an element measured in amu (atomic mass units), whereas Mass Number (Symbol: A also called "Nucleon Number" or "Atomic Mass Number") is simply the sum of the number of protons and neutrons in an atom of an element. It has no units because it is simply a number. It is calculated as A=Z plus n, where n is the number of neutrons. It is calculated like this because Z (the number of protons) and n (the number of neutrons) is equal to A (Atomic Mass , The sum of protons and neutrons.) For example: Beryllium has 4 protons and 5 neutrons so 4 plus 5 = 9. So, it's Mass Number = A = 9. Similarly, Helium has 2 protons and 2 neutrons. 2 plus 2 = 4. So, it's Mass Number = 4.
Example: How many protons and neutrons are in an atom having 238 mass number and 92 atomic number?
Solution:
Data:
A = 238
Z = 92
As we know that Z = number of protons. So,
Number of protons = Z = 92 (Found)
Number of neutrons = ?
= A - Z
=238 - 92
= 146 (Found)
So, such an atom contains 146 neutrons and 92 protons. Answer.
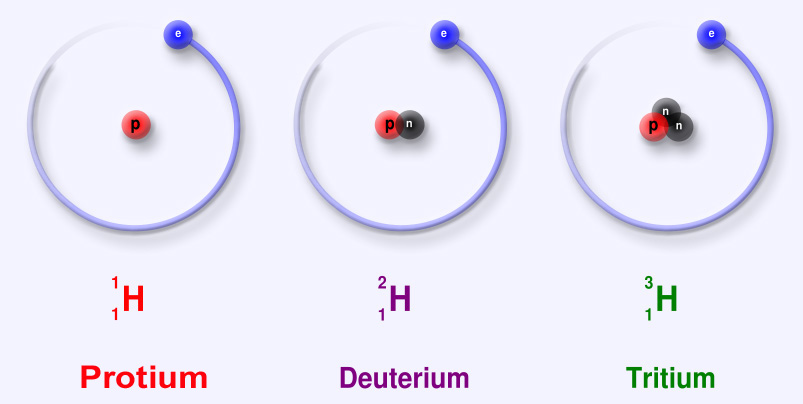
Isotopes:
There are version of some atoms. An element having same atomic number. But, different mass number is called an Isotope. For example: There are 3 isotopes of Hydrogen namely Protium, Deuterium and Tritium. Protium (normal hydrogen) has 1 proton and no neutrons, deuterium has 1 proton and 1 neutron & in tritium there are 2 neutrons and 1 proton. Similarly, there are 3 isotopes of Carbon. 2 are stable and 1 is radio-active (emits radiations). Carbon-12 (Normal carbon) has 6 protons and 6 neutrons, Carbon-13 has 6 protons and 7 neutrons & Carbon-14 has 6 protons and 8 neutrons. The Carbon-12 isotope is found 98.9% in nature. Carbon-13 and Carbon-14 are found 1.1% in nature.
Application of Isotopes:
Radiotherapy:
For the treatment of skin cancer, P-32 and Sr-90 are used because they emit less penetrating beta radiations. For the treatment of cancer, Cobalt-60 is used because it emits strongly penetrating beta radiations.
Tracer for Diagnosis & Medicine:
Iodine-131 is used to diagnose the presence of tumor and technitium is used to monitor bone growth.
Archaeological & Geological Uses:
The radioactive isotopes can be used to estimate the age of dead fossils e.g. dead plants & animals. Estimating the age of old objects is called Radio-Active Isotope Dating. If done with Carbon, it is called Carbon Dating OR Radio-Carbon Dating. Carbon-14 is used for this purpose.
Chemical Reaction & Structure Determination:
The radio-isotopes are used in a chemical reaction to follow a radio-active element during the reaction and ultimately to determine the structure. For example: Carbon-14 is used to label Carbon Dioxide. As Carbon Di-Oxide is used by plants for photosynthesis (Photo means light and synthesis means making. So it means "making (plant food) with light). to form glucose, it's movement is detected through the various intermediate steps up to glucose.
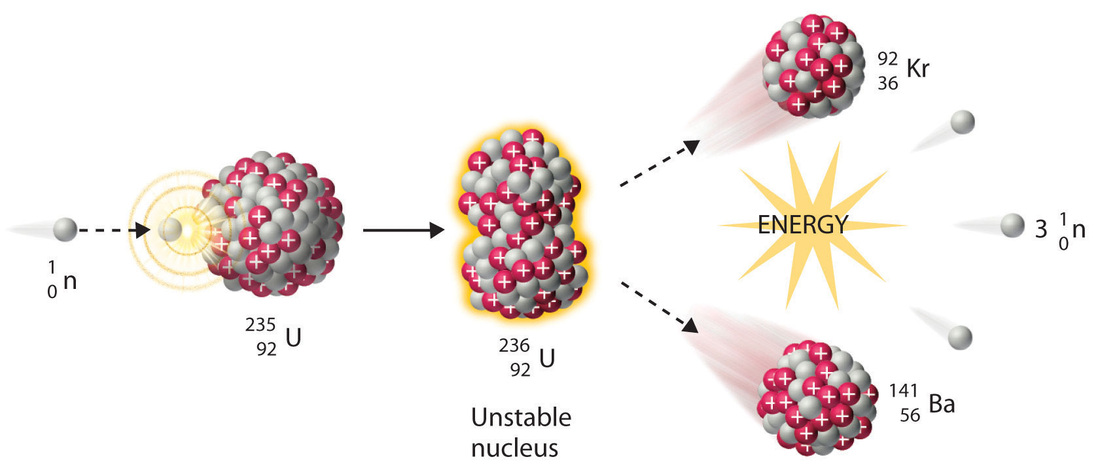
Applications in Power Generations:
Radioactive isotopes are used to generate electricity by carrying out controlled nuclear fission reactions in nuclear reactors. For example, when Uranium-235 is bombarded with slow moving neutrons, the Uranium nucleus breaks up to produce Barium-139, Krypton-94 and 3 neutrons.
A large amount of energy is released which is used to convert water into steam in boilers. The steam then drives the turbines to generate electricity. This is the peaceful use of atomic energy for the development of a nation.
 |
| Atomic Number And Mass Number |
 |
| Hydrogen's Isotopes |
 |
| Carbon's Isotopes |
 |
| Production of Kr-92, Ba-141, Energy & 3 neutrons. |
No comments:
Post a Comment