Matter:
Anything that has mass (i.e. occupies/takes space) is called "Matter". We and most of the things around us are matter. Matter is of mainly 4 states: Solids, Liquids, Gases and Plasma.
Substance:
Pure matter is called "substance". It has specific properties and characteristics. Elements (homo/same atoms) are substances.
Mixture:
Impure matter is called "mixture" OR When 2 or more elements or compounds mix without the atoms sticking together, a mixture is formed. It shows the properties of the constituents. The things in a mixture can be separated by simple physical methods e.g. distillation, filtration, evaporation, crystallization & magnetization.
Types:
It has 2 types:
Homogeneous Mixture:
If a mixture has uniform (same) composition throughout, it is called a "Homogeneous Mixture". e.g. air, gasoline and ice-cream. For example: Air is a mixture of nitrogen, oxygen, carbon dioxide, noble gases and water vapors.
Heterogeneous Mixture:
If a mixture does not have uniform composition throughout, it is called a "Heterogeneous Mixture". e.g. soil, rock & wood. For example: Soil is a mixture of sand, clay, minerals, water, salts and air.
Separation of constituents of
Mixture:
Let us consider an example. Suppose there are a few stones in some water. If the water is filtered, the stones are separated from the water. Filtration is a simple physical method. So, the constituents of mixture can be separated by simple physical methods.
 |
| Classification of Matter |
 |
| Homogeneous & Heterogeneous Mixtures |
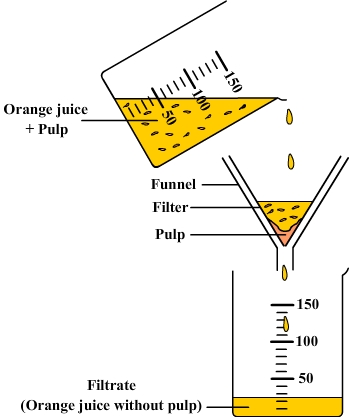 |
| Filtration |
No comments:
Post a Comment